Our Science
ShilpaBio has innovated unique platforms positioned to provide business value to customers worldwide
Case Study I – Process Development for a Sialylated Therapeutic Fc Fusion Protein
Process Development for a Sialylated Therapeutic Fc Fusion Protein
| Background | Program Complexity | Program Accomplishments | Conclusions |
|---|---|---|---|
|
|
|
|
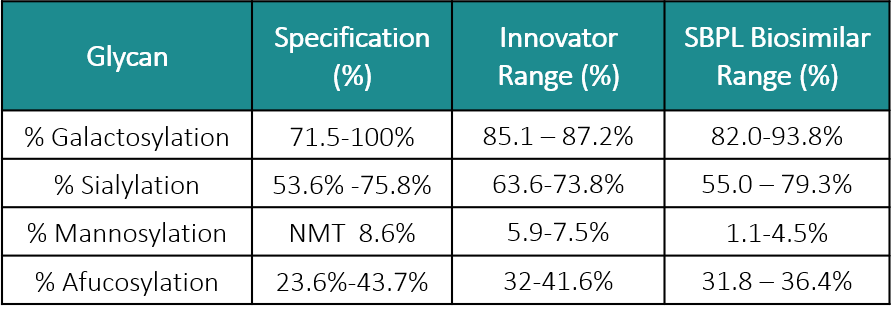
Overlapping glycan range is observed in SBPL and Innovator product
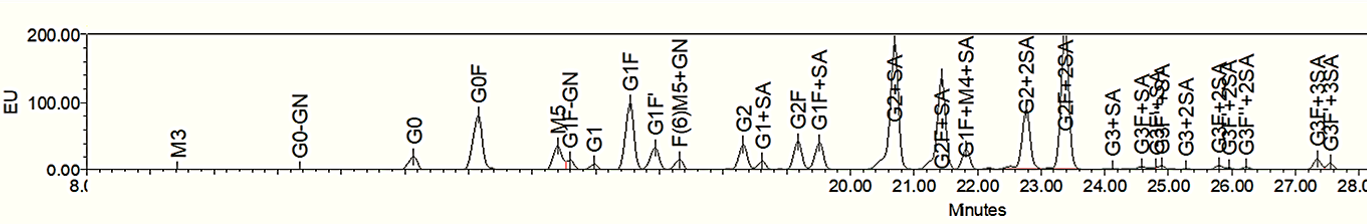
Glycan Profiling by UPLC Analysis
We have developed an Fc fusion protein with high biosimilarity to the innovator. Our unique perfusion strategy has been developed to achieve the highest PCD and titers along with preservation of bioactivity
Case Study II – Process Development for Enhancing Yields for an Fc Fusion Protein
Process Development for Enhancing Yields for an Fc Fusion Protein
| Background | Program Complexity | Program Accomplishments | Conclusions |
|---|---|---|---|
|
|
|
|
- We have developed an optimal fed batch process for the production of receptor-Fc Fusion protein
- We adopted a modified feeding strategy for achieving high PCD (per cell density) and titres
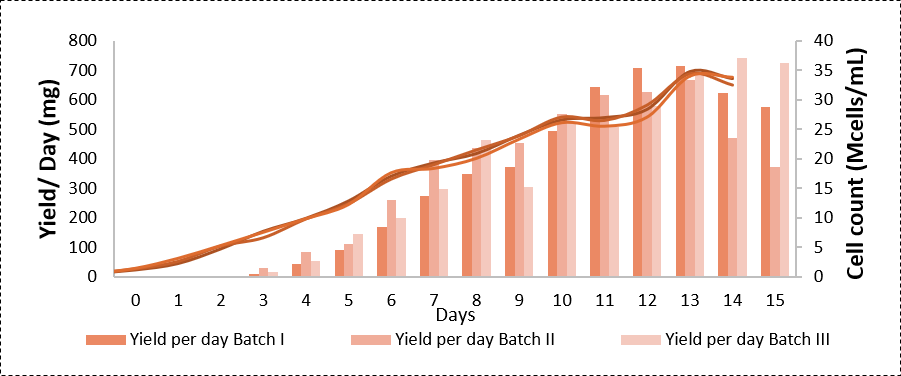
Cumulative yield per batch – 8 g/L in 15 days
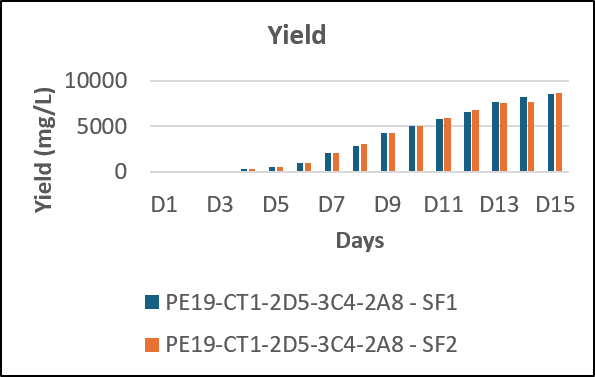
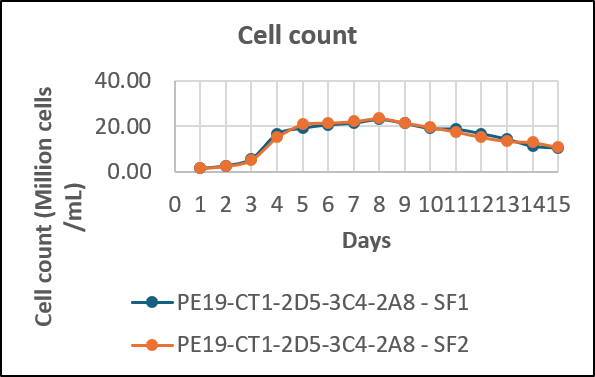
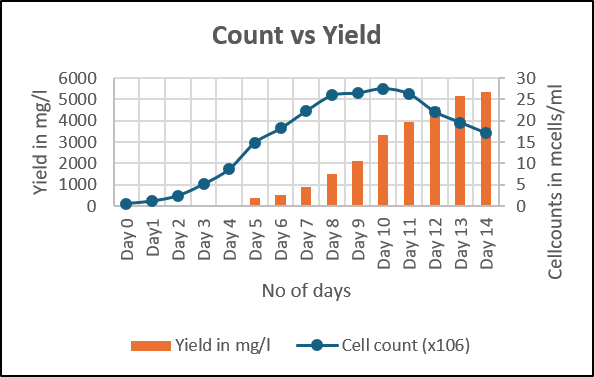
We have developed a receptor Fc fusion protein with high PCD and titers by using fed batch technology with highly optimized cost and time requirements as compared to other manufacturers
Case Study III: Covid-19 Sputnik Vaccine Development and Manufacturing
Covid-19 Sputnik Vaccine Development and Manufacturing
| Background | Program Complexity | Program Accomplishments | Conclusions |
|---|---|---|---|
|
|
|
|
We demonstrated the ability to modify our facilities and scale up our capacity with speed to meet the demands of a national public health emergency under full compliance to global regulatory standards
Case Study IV: New Biological Entity Development and Manufacturing
New Biological Entity Development and Manufacturing
| Background | Program Complexity | Program Accomplishments | Conclusions |
|---|---|---|---|
|
|
|
|
We demonstrated the ability to modify our facilities and scale up our capacity with speed to meet the demands of a national public health emergency under full compliance to global regulatory standards
We welcome partners who share the mutual goal of making high quality biopharmaceuticals affordable to patients globally.
Please write to [email protected] with your queries.


